8+ Chapter 13 Review Ions In Aqueous Solutions And Colligative Properties
Acids yield hydrogen ions in aqueous solutions. Click here to access free chemistry study material.

Chem Ch 13 Ions In Aqueous Solutions And Colligative Properties Flashcards Quizlet
Sodium is the cation.

. We need to make a few more reasonable assumptions or approximations. Solutions colligative properties are properties that depend on the concentration of molecules or ions of the solute but not on the. In this solution an excess of solid AgCl dissolves and dissociates to produce aqueous Ag and Cl ions at the same rate that these aqueous ions combine and precipitate to form solid AgCl Figure 152.
NCERT Solutions for Class 10 Science. Discuss next the naming of acids. NCERT Solutions for Class 10 Science.
Ions in Aqueous Solutions and Colligative Properties. Drugs are rarely administered as pure chemical substances alone and are almost always given as formulated preparations or medicines. NCERT Solutions for Class 10 Maths Chapter 14 More.
Go to Holt McDougal Modern Chemistry Chapter 13. The relationship between nutrition and health was summarized in Figure 12 illustrating that the nutritional quality and quantity of foods eaten and therefore nutritional status are major modifiable factors in promoting health and well-being in preventing disease and in treating some diseases. Kinetics 6 hours lectures 3 hours seminars.
Online Textbook Help Course Holt McDougal Modern Chemistry Chapter 13. In the previous example if table salt NaCl were added to the solution before or after adding eqPbCl_2 eq less would dissolve into the water because there would be chloride ions more. Shift In Equilibrium Between Ferric Ions And Thiocyanate Ions Experiment.
Convert the following to the appropriate number of significant figures. Titration is based on a reaction between the analyte unknown sample and the regent of known concentration and reaction stoichiometry. NCERT Solutions for Class 10 Maths Chapter 14.
Overcome problems arising during preparation of pharmaceutical solutions. These can vary from relatively simple solutions to complex drug delivery systems through the use of appropriate additives or excipients in the formulations. The first part of the name starts with the prefix hydro- followed by the name of the element modified by the ending ic.
Find step-by-step solutions and answers to Modern Chemistry - 9780547586632 as well as thousands of textbooks so you can move forward with confidence. Sigma And Pi Bond. SAT Subject Test.
Many drugs are formulated as solutions or added as powder or solution forms to liquids. NCERT Solutions for Class 10 Maths Chapter 13. A hydroponic nutrient solution is described as an aqueous mixture that contains inorganic ions from soluble salts of essential nutrients for plants along with certain organic substances like iron.
An earlier chapter of this text introduced solutions defined as homogeneous mixtures of two or more substancesOften one component of a solution is present at a significantly greater concentration in which case it is called the solventThe other components of the solution present in relatively lesser concentrations are called solutesSugar is a covalent solid composed of. Have information about the structure and intermolecular forces of the drug. The equation shows that even though they started with a solid it has broken apart into liquid aqueous ions.
Reverse osmosis is the process in which pressure is applied to overcome colligative property and osmotic pressure that is. NCERT Solutions for Class 10 Maths Chapter 14. Norton Company Inc.
Colligative properties are dependent on the amount of a solute but are not. 126 Colligative Properties of Electrolyte Solutions. Relative lowering of vapour pressure boiling point elevation and boiling point depression in binary solutions containing non-volatile solutes.
NCERT Solutions for Class 10 Maths Chapter 13. If relatively little ionization occurs the acid or base is weakAs will be evident throughout the remainder of this chapter there are many more weak acids and bases. Acid and Base Ionization Constants.
Reaction kinetics in relation to reaction mechanism. If the ionization reaction is essentially complete the acid or base is termed strong. Because silver chloride is a sparingly soluble salt the equilibrium concentration of its dissolved ions in the solution is relatively low.
Drugs with low aqueous solubility often present problems related to their formulation and. C12H22O11-1 NaCl-2 CaCl2-3 C2H6O-1 Na2SO4-3. NCERT Solutions for Class 10 Science.
Binary acids composed of hydrogen and another element usually a nonmetal. H2S hydrosulfuric acid iii. Go to Holt McDougal Modern Chemistry Chapter 8.
An acid-base reaction is one in which a hydrogen ion H is transferred from one chemical species to anotherSuch reactions are of central importance to numerous natural and technological processes ranging from the chemical transformations that take place within cells and the lakes and oceans to the industrial-scale production of fertilizers. In more basic solutions where the hydronium ion concentration is less than 50 10 9 M pH 83 it is red or pink. It is used to remove ions minerals chemicals and impurities from water.
Vant Hoff explained that when solutes are dissolved in a solvent they dissociate into ions. The theoretical values of molecular mass when calculated from the colligative properties of solutions are sometimes found to differ from the experimentally obtained valuesThese values are often referred to as abnormal molar masses. TITRIMETRIC MTHODS Titrimetric methods are widely used in chemistry to determine oxidants reductants acids bases metal ions etc.
Learn about Chemistry its branches and the key concepts covered under the subject at the K-12 level. Colligative Properties Relative Lowering Of Vapour Pressure. Chemistry is the study of matter its properties how and why substances combine or separate to form other substances and how substances interact with energy.
136 meters to inches. The relative strength of an acid or base is the extent to which it ionizes when dissolved in water. Ions in Aqueous Solutions and Colligative Properties Chapter.
Since the solution is aqueous we can proceed as if it were water in terms of its specific heat and mass. Identify the ideal value of the vant Hoff factor for aqueous solutions of each solute. Science Courses Holt McDougal Modern Chemistry.
One with a positive charge and the other with a negative charge. 300 cm 3 of an aqueous solution contains 156g of a polymer. For example phenolphthalein is a colorless substance in any aqueous solution with a hydronium ion concentration greater than 50 10 9 M pH 83.
NCERT Solutions for Class 10 Maths Chapter 13. HI hydroiodic acid a.
Chapter 13 Ions In Aqueous Solutions And Colligative Properties Welcome To Salomon S World Of Chemistry

Ions In Aqueous Solutions And Colligative Properties Ppt Video Online Download

Colligative Properties
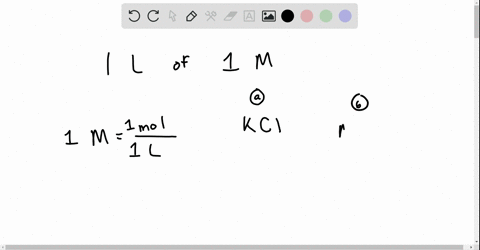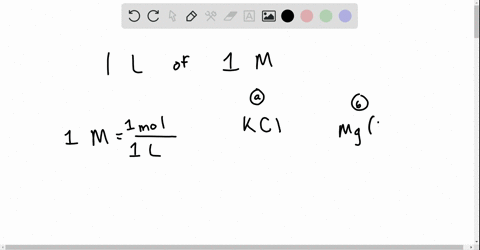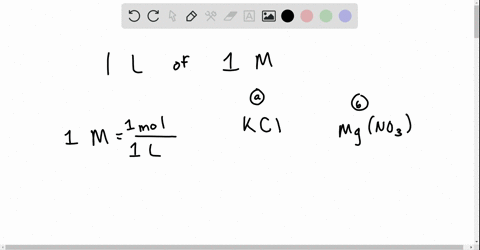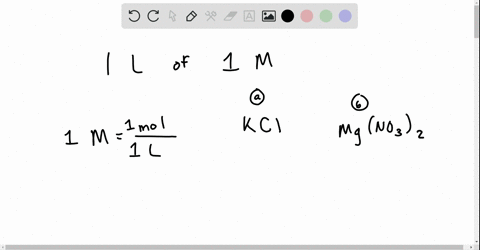
Chapter 13 Ions In Aqueous Solutions And Colligative Properties Video Solutions Holt Modern Chemistry Numerade

Chapter 13 Lecture On Solutions Colligative Properties

Chapter 13 Preview Multiple Choice Short Answer Ppt Video Online Download

Chapter 13 Ions In Aqueous Solutions And Colligative Properties Video Solutions Holt Modern Chemistry Numerade

Chapter 13 Ions In Aqueous Solutions And Colligative Properties Video Solutions Holt Modern Chemistry Numerade

Ch 13 Review
Colligative Properties Raoult S Law Equations Examples Video Lesson Transcript Study Com

Ions In Aqueous Solutions And Colligative Properties

Chapter 13 Ions In Aqueous Solutions And Colligative Properties Ppt Download
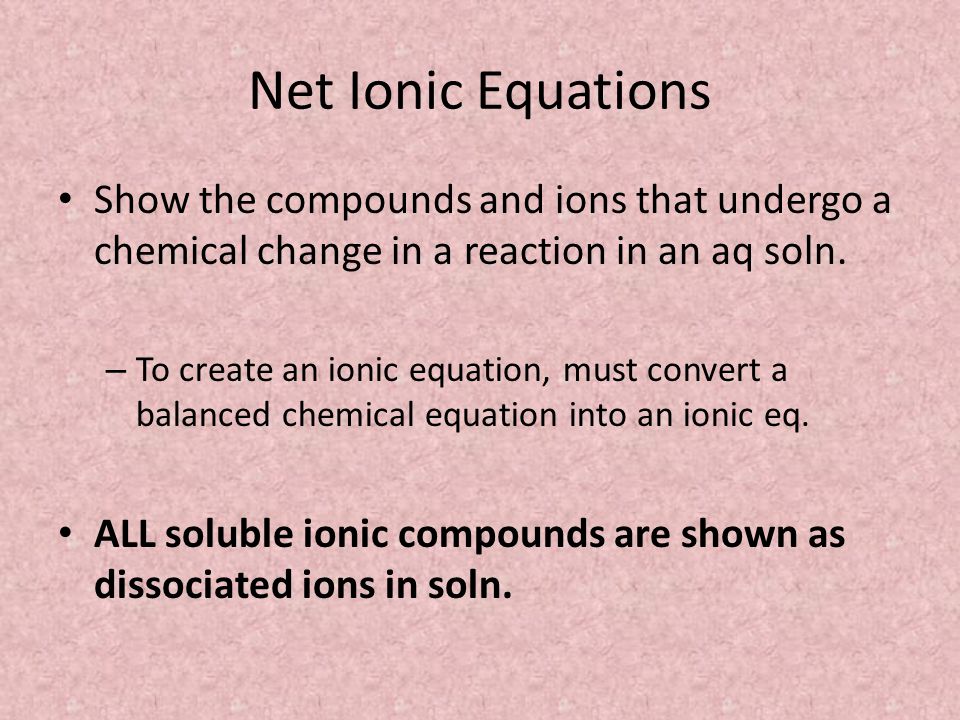
Ions In Aqueous Solutions And Colligative Properties Ppt Video Online Download

Chapter 13 Review Ions In Aqueous Solutions Fill Online Printable Fillable Blank Pdffiller

Colligative Properties

Colligative Properties Definition Types Examples Raoult S Law

Ch 13 Review